Structure and Location for Synapse - Lab Review
Introduction
Invaginating structures are small outward projections institute in a diverse array of cell types (Bastiani and Goodman, 1984; Petralia et al., 2015; Wood et al., 2021), including synapses of neurons of almost all animals (reviewed in Petralia et al., 2015, 2016, 2017, 2018). The invaginating structures involve prison cell membranes of ii different cells, with the outward projection – the invaginating structure – from i cell being surrounded by the invaginated membrane of the other cell. Therefore, in cross-sectional views of transmission electron microscopy (TEM), the invaginating structures can appear as double membrane-covered vesicles. In neuronal synapses, the invaginating structures can exist divided into two primary groups depending on the presence or absenteeism of active zones.
Invaginating structures tin be important in synapse physiology, still they ofttimes have been overlooked in studies of synaptic function. This is especially true for the smaller spinule types of invaginating structures because they are difficult to place without TEM, and fifty-fifty with standard 2D TEM, the origins of the invaginating structures are oftentimes obscure. Today, super-resolution and other specialized low-cal microscopy techniques allow amend visualization of these invaginating structures in synapses (Ueda and Hayashi, 2013; Zaccard et al., 2020). Moreover, the new moving ridge of 3D EM methods such as focused ion beam-scanning electron microscopy (FIB-SEM) makes tracing of these invaginating structures possible. These approaches are inspiring scientists to examine the role of invaginating structures in synapses and neurons. In this perspective, nosotros describe some of the more than interesting examples of invaginating structures including several new examples from across the creature kingdom. We also talk over the latest ideas about how they may be cardinal to the regulation of synaptic and neuronal office.
Results and Discussion
Invaginating Structures Associated With Mechanoreception and Photoreception (Figure 1A)
Some of the most elaborate arrangements of invaginating structures are found in synapses of the circuits involved in processing mechanoreception or photoreception and are adaptations to allow animals to respond very rapidly to irresolute environmental mechanical and visual stimuli (Petralia et al., 2017). They include diverse combinations of invaginating presynaptic terminals and postsynaptic spines (Figure 1A). The most amazing example is seen in cubozoan jellyfish, which have optics as elaborate as those of higher animals fifty-fifty though they lack brains! These jellyfish exhibit circuitous behaviors involving vision, such as fugitive obstacles, prey capturing, and complex mating behaviors (eastward.one thousand., Nilsson et al., 2005). They possess photoreceptor cells with prominent invaginating spines from postsynaptic cells or other photoreceptor cells (Grey et al., 2009). This suggests that the invaginating synapse was one of the earliest functional developments in animate being nervous systems, even forming prior to the evolution of whatsoever form of "encephalon." Invaginating postsynaptic spines can exist found in some invertebrate sensory cell synapses such equally in the octopus statocyst involved in balance and hearing, and mechanoreceptor cells involved in the defensive gill-withdrawal reflex of the ocean hare, Aplysia (Bailey and Thompson, 1979; Bailey et al., 1979). Interestingly, the invaginating spines of Aplysia have twice equally many presynaptic vesicles equally non-invaginating ones; the authors attribute this to the loftier degree of synaptic plasticity related to the reflex (Bailey and Thompson, 1979; Bailey et al., 1979). Pilus cell synapses of the tunicate, Ciona intestinalis, can have invaginating structures at their base and these can be postsynaptic, presynaptic, or both (reciprocal – with presynaptic vesicles on both sides of the synapse; Rigon et al., 2018). In the octopus (Figure 1A), the photoreceptor terminals form large bag- or carrot-shaped structures that are filled with presynaptic vesicles and contain (1) invaginating postsynaptic spines, (2) presynaptic vesicle-filled "finger twigs" from next carrots, and (three) "tunnel fibers" from pocket-sized neurons (Dilly et al., 1963; Instance et al., 1972). Structures like "finger twigs" also are found in squid photoreceptor terminal "carrots." Neither the finger twigs nor tunnel fibers show any distinctive signs of chemic synapses (no definitive active zones with densities), except for the synaptic vesicles in the finger twigs. Due to their deep invagination of the photoreceptor terminal, these structures are instead ideally bundled to mediate electrical field/ephaptic conduction (Cohen, 1973; Haghighat et al., 1984; Petralia et al., 2017).
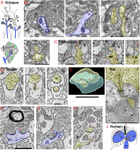
Figure 1. Invaginating structures are common in animal synapses. (A) Drawings recapitulating the octopus's large en passant photoreceptor terminals, chosen "numberless" (b) or "carrots." The bags are filled with synaptic vesicles (shown in lower cartoon) and incorporate iii types of invaginating structures from three different sources, including: (1) postsynaptic spines (bluish) with a dense layer of synaptic vesicles surrounding the deeply invaginating spine heads; (2) presynaptic terminals, besides called "finger twigs" (f), which are filled with synaptic vesicles (lower cartoon), invaginating from adjacent bags; and (3) "tunnel fibers" (t), which are one or more nerve trunks passing in a "tunnel" through the bag at ∼right angles to the invaginating spines and originating from small neurons called "microneurons." Mitochondria are green. Drawings are from Petralia et al. (2017) with slight modifications. (B) Electron microscopy (EM) images of the planaria encephalon synapses. The invaginating structures include an invaginating postsynaptic dendrite (blue, left prototype), an invaginating filopodium (f, centre image), and interdigitating axon terminals (yellow and uncolored, right epitome). In the EM image on the left in the 2nd row, an unidentified projection invaginates into an axonal terminal (yellow) with large dense-cored vesicles. (C) EM images show an invaginating construction from the ventral ganglion of the nematode, Pristionchus pacificus (Bumbarger et al., 2013; serial cross-section online information set in Neurodata OCP). An invaginating structure (asterisk) originates from an axon terminal (yellow), which is one of two vesicle-filled terminals that class typical nematode dyadic synapses with a presynaptic density (arrows) centered between two postsynaptic processes (lacking PSD; White et al., 1986; Hall and Russell, 1991). The invaginating procedure enters into the base of a neurite extending from a neuron soma of the ventral ganglion (cell matches descriptions of neurons by position and structure; Ware et al., 1975; White et al., 1986). A possible junction may occur on the dorsal attribute of the invaginating procedure where the membranes appear denser and in that location are unidentified subsynaptic structures (arrowheads) in the postsynaptic cell. The left two images are transverse sections (z positions 2017 and 2019 in the image stack), and the right image is a digitally reconstructed parasagittal section. (D–H) Invaginating structures in the mouse nucleus accumbens. (D) An invaginating presynaptic terminal (yellow). The z positions in the FIB-SEM image stack are 144, 202, and 237 for the three images. The master part of the terminal partly invaginates into the cup-shaped postsynaptic process, and information technology then invaginates a portion of the concluding deep within the postsynaptic process (asterisk). (E) A 3D reconstruction of a similar invaginating presynaptic (yellow) last (asterisk) from the aforementioned data set in console (D), turned virtually 90 degrees relative to the construction in panel (D). The postsynaptic membrane also invaginates a brusque spinule (arrow) into the presynaptic concluding (yellow), like to the ane shown in console (F). The 3D reconstruction is reprinted, afterwards slight modification, from Delgado et al. (2019). (F,Grand) Examples of postsynaptic (blue) membrane invaginating short spinules (arrows) into presynaptic terminals. The EM paradigm in panel (F) also includes a myelinated axon in which the glial cytoplasm (oligodendrocyte) partly invaginates into the axon. (H) Two presynaptic terminals invaginate short spinules (arrows) into dendrites (adjacent EM image in z position to this EM image is published in Delgado et al., 2019). (I) ImmunoEM of rat brain synapse. Immunogold localization (arrows) of GABA-A receptors in invaginating structures in the rod spherule of the rod photoreceptor synaptic terminal circuitous (r) in the rat retina. As is typical in vertebrate retinas, a complex of processes (b, h) from bipolar and horizontal cells invaginate into the terminal adjacent to the active zone identified by the presynaptic ribbon (asterisk). The immunogold labeling for GABA-A (pointer) is concentrated between a horizontal cell procedure and a small projection extending from the presynaptic rod cytoplasm and directly subjacent to the active zone. (J) Drawing shows that in the human retina, rod photoreceptor synaptic terminals accept a ribbon (asterisk) synapse with an invaginating structure from one bipolar and two horizontal cells (b, h) plus a small projection of cytoplasm from the rod terminal. Horizontal cell processes can form synapses (red arrows) with the rod last and its projection and with the bipolar cell process; they comprise large vesicles and presynaptic densities (Linberg and Fisher, 1988). Panels (I,J) are reprinted from Petralia et al. (2017) with slight modifications. Scale bars (B,I) = 500 nm, (C,E,F) (use D,G,H) = 1 μm.
Simple Brains (Figures 1B,C)
Flatworms are the simplest animals with bilateral symmetry, a head, and a brain. Even at this primeval stage in brain evolution, a variety of invaginating structures are evident including at postsynaptic dendrites or other cellular processes with or without synaptic active zones, and various presynaptic terminals invaginating and interdigitating with other terminals (Figure 1B; Petralia et al., 2015). Nematodes have a simple nervous system with a minimal "brain" construction equanimous of a circumpharyngeal nerve ring and associated neuron clusters including the ventral ganglion (White et al., 1986). Recent studies show that nematodes take a variety of types of spine synapses similar to those found in vertebrates (Cuentas-Condori et al., 2019). White et al. (1986) showed several examples of presynaptic terminals invaginating into postsynaptic processes, and postsynaptic processes (spines) invaginating into presynaptic terminals, as well equally a motoneuron last invaginating into an interneuronal cell body. In Figure 1C, a presynaptic concluding invaginates a structure into the base of a neurite extending from a neuronal soma in the ventral ganglion. A possible junction may occur on the invaginating structure where the membranes appear denser and there are unidentified subsynaptic structures in the postsynaptic jail cell.
Vertebrate Brains (Figures 1D–H)
Invaginating structures are rather common in synapses of the vertebrate encephalon. For example, in a contempo report of the human temporal cortex, Rollenhagen et al. (2020) found examples of postsynaptic spines invaginating into presynaptic terminals. They also establish examples of presynaptic terminals with active zones and large non-synaptic structures from presynaptic terminals, both of which invaginate into dendrites. Nosotros have examined a FIB-SEM dataset from mouse nucleus accumbens showing various examples, including (1) postsynaptic spinules invaginating into presynaptic terminals, (2) invaginating structures from presynaptic terminals forming cup-shaped synapses with a more securely invaginating portion, and (3) short presynaptic spinules invaginating into dendrites (Figures 1D–H). These will be discussed beneath in relation to the published literature.
Spinules from the postsynaptic spine invaginating into the presynaptic terminal (Figures 1E–G) accept been described in many areas of the mammalian brain especially in the hippocampus (Westrum and Blackstad, 1962; Spacek and Harris, 2004; Yao et al., 2005; Tao-Cheng et al., 2009). An interesting example was documented in mouse barrel cortex, where some postsynaptic spines invaginate fully into the presynaptic terminals and then appear to extend a thick procedure, filled with various vesiculate structures and filaments, deeper within the terminal (Rodriguez-Moreno et al., 2018, 2020). Spinule formation is enhanced in hippocampal slice cultures following stimulation to induce long-term potentiation (LTP; Tao-Cheng et al., 2009) suggesting that spinules recycle extra postsynaptic membrane formed during enhanced synaptic activity. Indeed, some spinules are associated with the formation of the large, mushroom-shaped spines during synaptic plasticity such as that following LTP (Petralia et al., 2014, 2015, 2018). These mushroom-shaped spines enlarge since more than membrane is added as boosted glutamate receptor molecules are incorporated into the postsynaptic membrane; this increase in receptors likely enhances synaptic transmission. Plainly, this added membrane causes the PSD to become perforated in correlation with the increased density of glutamate receptors (Ganeshina et al.,2004a,b). At this signal, a spinule may class at the perforation, invaginate into the presynaptic terminal (Figure 1F), and transfer excess postsynaptic membrane into the presynaptic terminal (Spacek and Harris, 2004; Tao-Cheng et al., 2009; Petralia et al., 2014, 2015, 2018). Coated pits frequently are seen at the ends of spinules (Westrum and Blackstad, 1962; Spacek and Harris, 2004; Yao et al., 2005; Tao-Cheng et al., 2009), mediating removal and absorption of spinule ends into the terminal. And recent studies with enhanced resolution 3D low-cal microscopy have confirmed that neuronal activity induces spine-derived spinule elongation (Zaccard et al., 2020).
Invaginating structures originating from presynaptic terminals in many animals vary from small spinules (Figure 1H) to larger structures and are often filled with presynaptic vesicles (Figures 1D,E). In the mammalian forebrain, some spinules that invaginate into presynaptic terminals originate from adjacent axons or presynaptic terminals, from ∼12% in the CA1 region of the rat hippocampus (Spacek and Harris, 2004) to ∼35% in the visual cortex of the ferret (Campbell et al., 2020). Invaginating structures from side by side presynaptic terminals that are filled with synaptic vesicles ofttimes enter each other; these "pseudopodial indentations" or "PSIs" are described in some vertebrate synaptic terminals and can sometimes form complex intertwinings (Boyne and Mcleod, 1979; Boyne and Tarrant, 1982; come across invertebrate examples in Figures 1B, 2). Such complex structures could act as "variable improvidence traps" to control levels of ions and other substances in the space betwixt the processes (Boyne and Tarrant, 1982). Electrical stimulation of presynaptic terminals on the electrical organ of torpedo rays increases PSI frequency and size (∼27×; Boyne and Mcleod, 1979). Some inhibitory GABAergic terminals in the mammalian forebrain invaginate short structures into the postsynaptic cell. The postsynaptic membrane surrounding the invaginating structure contains an enzyme to synthesize cannabinoid that mediates a retrograde signal for tonic inhibition of synaptic activity (Yoshida et al., 2011; Omiya et al., 2015).
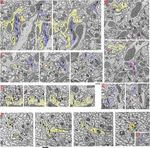
Effigy 2. Invaginating structures in the Drosophila brain. Examples are FIB-SEM image stacks of the protocerebral span (A–E) and mushroom trunk (F,G). Blue, dendrite; yellow, axon; magenta, either dendrite or axon or both. Axon terminals were defined by accumulation of synaptic vesicles or were traced to a presynaptic T-bar; dendrites were traced to a postsynaptic process. Invaginating structures are defined every bit outward projections. (A) Neurites 1 and two are large axon terminals that co-invaginate (neurite 1 invaginates into neurite 2 while neurite two invaginates into neurite 1). Neurite three is a dendrite that invaginates into axon 1, and neurite three is one of the two postsynaptic processes of a T-bar synapse (t) of axon 2. Neurite iv is a dendrite that invaginates into a glial cell process; neurite four also is 1 of two postsynaptic processes at a T-bar synapse in an adjacent axon terminal (left image). (B) Neurite 1 is an axon terminal that invaginates into an next axon last; neurite 1 is also postsynaptic at a T-bar synapse in the adjacent last (bottom image). Neurites 2, 3, and 4 invaginate into the same large axon terminal; iii and 4 are small dendrites. Neurite ii (magenta) was traced for a long distance (>4 μm). This neurite 2 displays features of both presynaptic and postsynaptic structures and forms at to the lowest degree two or three T-bar synapses as well as ii or three postsynaptic processes with different synapses (non shown). (C) Axon concluding 1 is invaginated past axon terminal 2 and also invaginates another terminal. Neurite 3 is a dendrite that forms four spine-like structures, including ane that forms a postsynaptic procedure at a synapse with terminal i and another that invaginates into a subjacent terminal. Neurite 4 is a dendrite that also invaginates into the same subjacent terminal. (D) A structure from axon terminal ii invaginates into axon terminal 1, while structures from dendrites 3, four and 5 invaginate into terminal ii. (E) Neurites 1, 2, and 3 are projections from dendrites that invaginate into the same large axon terminal; neurite 1 has ii invaginating structures. Neurite 3 too bears some T-bar like structures (not shown). Invaginating structures from axon terminals tin exist filled with synaptic vesicles every bit seen in panels (A,B), or devoid of vesicles as evident in panel (D). (F) Neurites 1-6 are all small axon terminals with relatively few synaptic vesicles. These axons invaginate with each other and besides often cluster to form synapses on central dendrite processes. (G) An instance of a dendrite (1) invaginating into an axon concluding. The number in the lower left or lower right corner of each micrograph indicates its z position in the FIB-SEM image stack. t = T-bar (only selected ones are labeled). Scale bars are 500 nm for panels (A–E) and (F–G). Annotation that the protocerebral span neuropil (A–E) contains arable invaginating processes from large axon terminals and dendrites, while the mushroom trunk neuropil (F,G) contains abundant invaginating processes from pocket-size axon terminals only few from dendrites.
Loving cup-shaped spines are highly concave spines that wrap around partly or fully invaginating presynaptic terminals. They are mutual in cerebral cortex and hippocampus of mammals, and specially in the dentate gyrus (Desmond and Levy, 1983; Frotscher and Leranth, 1986; Petralia et al., 2017, 2018). Cup-shaped spines tin be even more complex in the nucleus accumbens (Delgado et al., 2019; Yao et al., 2020), where the presynaptic last can keep in part as a deeper invagination with a synaptic agile zone (Figures 1D,E; Delgado et al., 2019). Desmond and Levy (1983) found that high-frequency stimulation of entorhinal cortex input increases the number of concave spines in the dentate gyrus. Spines in CA1 slice cultures appear more loving cup-like after chemical induction of LTP (Nagerl et al., 2008), while the number of loving cup-shaped spines decreases afterwards high-frequency electric stimulation to induce LTP in CA1 slice cultures (Chang and Greenough, 1984). Cup-shaped spines appear to be more than common in both slice and dissociated cultures compared to intact tissue (Roelandse et al., 2003; Mitchell et al., 2012; Petralia et al., 2017 and unpublished data). All of this suggests that germination of cup-shaped spines is a type of spine plasticity that is analogous in some means to evolution of the large convex mushroom spines.
Drosophila: Brain (Figure two) and Neuromuscular Junctions
One of the most striking recent revelations nigh invaginating structures in synapses has occurred for the insect brain. When we first reviewed the invaginating structures of all animals in 2015 (Petralia et al., 2015), such structures were almost unknown for the insect encephalon.
The only examples were glia-derived capitate projections invaginating into photoreceptor terminals in the Drosophila eye (Prokop and Meinertzhagen, 2006) and some interaxonal invaginating structures (Petralia et al., 2015). Then, in 2018, utilizing FIB-SEM, (Gruber et al., 2018) described the synaptic spinules of the olfactory circuit of the Drosophila brain, and it became apparent that synaptic spinules are common. As can exist seen for 2 areas of the Drosophila brain in Figure 2, there is a loftier abundance of invaginating neuronal processes into axonal terminals, derived from either dendrites or other axonal terminals. This design appears to be the rule for the Drosophila encephalon. Interestingly, some of the invaginating structures are derived from neurites with reciprocal synaptic functions, acting as both axon and dendrite. 1 such example is shown in Figure 2B: neurite 1 is a vesicle-filled axonal terminal but also forms one of the two postsynaptic elements of a photoreceptor terminal T-bar synapse, and neurite ii was traced to different portions (not shown) containing postsynaptic processes or presynaptic T-bars. Like reciprocal structures in interneurons are described for the ocellar photoreceptor terminal complex of Drosophila (Stark et al., 1989) that shows an example of a vesicle-filled interneuron invaginating into a photoreceptor terminal. Nonetheless, photoreceptor terminals in both compound optics and ocelli of Drosophila are invaginated mainly by specialized glial processes, rather than axonal or dendritic ones (reviewed in Prokop and Meinertzhagen, 2006; Petralia et al., 2015). Overall, the complexity of the invaginations in the Drosophila brain rivals or surpasses those found in the vertebrate brain, all the same these neuronal invaginations in insect synapses were overlooked or missed by electron microscopists for the past 60 years!
Invaginations from presynaptic terminals also are common at neuromotor junctions including neuromuscular (NMJ) and secretomotor (such as glands) junctions (Petralia et al., 2017). These invaginating structures tin either partially or fully invaginate into the postsynaptic cell. Such invaginating structures are function of mechanisms mediating rapid responses of skeletal muscle fibers. Because these invaginating structures also are found in NMJs of some slower muscles and glands, they might facilitate maintaining an enclosed space for exchange of regulatory factors. This role is best understood for NMJs of larval Drosophila skeletal musculus (reviewed in Deshpande and Rodal, 2016;, Van Vactor and Sigrist 2017, Guangming et al., 2020). A hundred-fold increase in muscle expanse occurs during larval growth (Deshpande and Rodal, 2016) and this must be accompanied by an equally impressive and matching growth in the NMJ; thus, this enclosed invagination area is a special arrangement to allow for the exchange back and forth across the synapse of a big number of dissimilar growth and regulatory factors to maintain this system through development. For example, Wg (wingless; a Wnt ligand) is ane of several regulatory proteins transported from the presynaptic final membrane via release of exosomes, probably from multivesicular bodies into the invagination intercellular space, that affect postsynaptic differentiation; other factors move retrogradely to affect presynaptic differentiation (Deshpande and Rodal, 2016). Some other curious instance is the transport of Arc1, important for synaptic plasticity, in capsid-like structures of Arc1 protein + mRNA within exosomes probably derived from presynaptic multivesicular bodies (Ashley et al., 2018).
Invaginating Complexes of Processes (Figures 1I,J)
Some mechanoreceptor and photoreceptor cells in various invertebrates and vertebrates accept large invaginations at their bases that contain a complex of both postsynaptic and presynaptic invaginating processes (Petralia et al., 2016, 2017). This is all-time known for the photoreceptor synapses of vertebrates (Figures 1I,J), in which the various processes are arranged within as well as subjacent to the invagination. Thus, they are in dissimilar positions and with unlike combinations of glutamate receptors within the area of glutamate spillover diffusion; GABA and ephaptic conduction are probably besides involved hither (Kramer and Davenport, 2015; Petralia et al., 2017). The primary invaginating structures extend from bipolar and horizontal cells; their invagination and function are partly dependent on trans-synaptic complexes of proteins including calcium channel subunits and receptors (Kerschensteiner, 2017; Wang et al., 2017; Cao et al., 2020; Maddox et al., 2020; Tsukamoto et al., 2021). Invaginating horizontal cell processes form a type of reciprocal synapse including a feed-forward part forth with negative feedback to provide lateral inhibition to help the brain modulate signals from groups of side by side photoreceptor cells. The feedback mechanism from the horizontal cell processes to the photoreceptor jail cell may involve variable combinations of 3 different mechanisms: GABA (Figure 1I), proton (H+), and ephaptic transmission (electrical coupling between nerve processes not involving direct synapses) (Liu et al., 2013; Gardner et al., 2015; Kramer and Davenport, 2015; Petralia et al., 2017; Barnes et al., 2020; Hirano et al., 2020).
Horizontal cell processes vary in structure amidst vertebrates, and often have large vesicles of unknown function. Human horizontal cell processes at the rod photoreceptor last grade definitive synapses (Figure 1J; Linberg and Fisher, 1988). Many fish have unusual spinules that invaginate into the photoreceptor cell from the horizontal cell processes, and they accept enlarged ends with internal densities (Popova, 2014). These structures are numerous in the day but mostly gone at night. Popova (2014) suggests that they mediate feedback activity essential for the coding of combative color information. They perhaps take some part in postsynaptic neurotransmission and retract when glutamate receptors are activated (Weiler and Schultz, 1993
Why Are Invaginating Structures So Of import for Synapse Function?
We have discussed the various aspects of this question in greater detail in our previous reviews (Petralia et al., 2015, 2016, 2017, 2018). This is peradventure easier to answer for those invaginations with synaptic active zones containing presynaptic vesicles and postsynaptic densities. In these cases, the invagination creates a unique, isolated environment for biochemical commutation/activity between the presynaptic and postsynaptic structures. Depending on the structural arrangements, this tin can either improve the manual of biochemical and/or electrical signals or sequester and isolate chemicals associated with plasticity between pre- and postsynaptic processes. One such case is the mossy concluding synapses of the hippocampus (Petralia et al., 2016, 2018). These large terminals are invaginated by large, modified chemical compound spines called thorny excrescences, providing numerous agile zones within the invagination (somewhat similar structures are found in the thalamus; Petralia et al., 2016; Pelzer et al., 2017). The crack region is continuous and excludes glial processes. Overall, this specialized synapse is designed to accept a higher net probability of release than typical cortical synapses (Henze et al., 2000). And as we accept discussed, the invagination in the retinal photoreceptor synapses is highly organized with processes bundled at unlike distances and positions to take best advantage of neurotransmitter spillover and feedback mechanisms to affect the highly specialized visual responses. In some cases, an invaginating process without active zones is designed to modify neurotransmission, equally we have discussed for presynaptic invaginating processes in inhibitory synapses in the mammalian forebrain and horizontal cell spinules in the fish retina. The Drosophila NMJ is the best studied case of a synaptic invagination providing an isolated and regulated local environment for chemical exchange to affect synaptic plasticity, as we discussed above. Finally, a large variety of small-scale invaginating processes exists, and which are often broadly classified equally "spinules," lacking agile zones and originating from postsynaptic, presynaptic, or glial components of the synapse. Many lines of evidence support various functions for these spinules in nutrient substitution, modulation/mediation of synaptic action, and interneuronal signaling. Well-nigh intriguing and least studied are possible electric field/ephaptic signaling furnishings (Faber and Pereda, 2018) that are likely facilitated past the invaginating structures (Gardner et al., 2015).
Data Availability Statement
The raw data supporting the conclusions of this article will exist made bachelor by the authors, without undue reservation.
Author Contributions
RP and PY organized the manuscript and figures. RP wrote the master text. RP, PY, and Y-XW provided the inquiry data for figures. PY and DK edited the manuscript. All authors reviewed the manuscript.
Funding
This study was supported by the Intramural Inquiry Programs of NIH/NIDCD and NIH/NIA. The Avant-garde Imaging Core code is ZIC DC000081.
Conflict of Interest
The authors declare that the research was conducted in the absenteeism of whatsoever commercial or financial relationships that could be construed every bit a potential conflict of interest.
Acknowledgments
We give thanks Harald F. Hess for providing the mouse nucleus accumbens FIB-SEM dataset, and Harald F. Hess and Shan C. Xu for providing the Drosophila protocerebral bridge and mushroom body FIB-SEM datasets. Nosotros as well thank Inna Belyantseva and Katie Kindt for critical reading of the manuscript.
Supplementary Fabric
The Supplementary Material for this commodity tin be found online at: https://www.frontiersin.org/articles/10.3389/fnsyn.2021.685052/full#supplementary-textile
References
Ashley, J., Cordy, B., Lucia, D., Fradkin, L. Thousand., Budnik, V., and Thomson, T. (2018). Retrovirus-like gag protein Arc1 binds RNA and traffics across synaptic boutons. Jail cell 172, 262–274. doi: x.1016/j.jail cell.2017.12.022
PubMed Abstract | CrossRef Total Text | Google Scholar
Bailey, C. H., and Thompson, E. B. (1979). Indented synapses in Aplysia. Brain Res. 173, 13–20. doi: 10.1016/0006-8993(79)91091-6
CrossRef Total Text | Google Scholar
Bailey, C. H., Thompson, Due east. B., Castellucci, V. F., and Kandel, E. R. (1979). Ultrastructure of the synapses of sensory neurons that mediate the gill-withdrawal reflex in Aplysia. J. Neurocytol. 8, 415–444. doi: ten.1007/bf01214801
PubMed Abstruse | CrossRef Full Text | Google Scholar
Barnes, S., Grove, J. C. R., McHugh, C. F., Hirano, A. A., and Brecha, Due north. C. (2020). Horizontal cell feedback to cone photoreceptors in mammalian retina: Novel insights from the GABA-pH hybrid model. Forepart. Cell Neurosci. 14:595064. doi: 10.3389/fncel.2020.595064
PubMed Abstract | CrossRef Full Text | Google Scholar
Bastiani, One thousand. J., and Goodman, C. S. (1984). Neuronal growth cone: Specific interactions mediated by filopodial insertion and induction of coated vesicles. P. Natl. Acad. Sci. 81, 1849–1853. doi: 10.1073/pnas.81.half dozen.1849
PubMed Abstract | CrossRef Full Text | Google Scholar
Boyne, A. F., and Mcleod, South. (1979). Ultrastructural plasticity in stimulated nerve-terminals - Pseudopodial invasions of abutted terminals in torpedine ray electric organ. Neuroscience 4, 615–624. doi: x.1016/0306-4522(79)90138-6
CrossRef Full Text | Google Scholar
Boyne, A. F., and Tarrant, Due south. B. (1982). Pseudopodial interdigitations between abutted nerve-terminals - Diffusion traps which occur in several nuclei of the rat limbic system. J. Neurosci. 2, 463–469. doi: 10.1523/jneurosci.02-04-00463.1982
PubMed Abstract | CrossRef Full Text | Google Scholar
Bumbarger, D. J., Riebesell, M., Rodelsperger, C., and Sommer, R. J. (2013). System-wide rewiring underlies behavioral differences in predatory and bacterial-feeding nematodes. Prison cell 152, 109–119. doi: 10.1016/j.cell.2012.12.013
PubMed Abstruse | CrossRef Total Text | Google Scholar
Campbell, C., Lindhartsen, Due south., Knyaz, A., Erisir, A., and Nahmani, M. (2020). Cortical presynaptic boutons progressively engulf spinules as they mature. Eneuro 7:5. doi: 10.1523/ENEURO.0426-19.2020
PubMed Abstruse | CrossRef Full Text | Google Scholar
Cao, Y., Wang, Y., Dunn, H. A., Orlandi, C., Shutlz, N., Kamasawa, N., et al. (2020). Coaction between cell-adhesion molecules governs synaptic wiring of cone photoreceptors. P. Natl. Acad. Sci. 117, 23914–23924. doi: 10.1073/pnas.2009940117
PubMed Abstract | CrossRef Full Text | Google Scholar
Instance, N. Grand., Young, J. Z., and Gray, E. G. (1972). Ultrastructure and synaptic relations in optic lobe of brain of Eledone and Octopus. J. Ultra Mol. Struct. R. 39, 115–123. doi: ten.1016/s0022-5320(72)80012-1
CrossRef Full Text | Google Scholar
Chang, F. L. F., and Greenough, Westward. T. (1984). Transient and enduring morphological correlates of synaptic action and efficacy alter in the rat hippocampal piece. Encephalon Res. 309, 35–46. doi: 10.1016/0006-8993(84)91008-4
CrossRef Total Text | Google Scholar
Cohen, A. I. (1973). Ultrastructural analysis of photoreceptors of squid and their synaptic connections .iii. Photoreceptor terminations in optic lobes. J. Comp. Neurol. 147, 399–425. doi: 10.1002/cne.901470306
PubMed Abstract | CrossRef Full Text | Google Scholar
Cuentas-Condori, A., Mulcahy, B., He, S. Westward., Palumbos, S., Zhen, Thousand., and Miller, D. M. (2019). C. elegans neurons take functional dendritic spines. Elife 8:47918. doi: 10.7554/eLife.47918
PubMed Abstract | CrossRef Full Text | Google Scholar
Delgado, T., Petralia, R. S., Freeman, D. W., Sedlacek, Thousand., Wang, Y. X., and Brenowitz, South. D. (2019). Comparison 3D ultrastructure of presynaptic and postsynaptic mitochondria. Biol. Open up 8:044834. doi: ten.1242/bio.044834
PubMed Abstruse | CrossRef Full Text | Google Scholar
Deshpande, M., and Rodal, A. A. (2016). The crossroads of synaptic growth signaling, membrane traffic and neurological illness: Insights from Drosophila. Traffic 17, 87–101. doi: 10.1111/tra.12345
PubMed Abstract | CrossRef Total Text | Google Scholar
Desmond, N. L., and Levy, West. B. (1983). Synaptic correlates of associative potentiation depression - an ultrastructural-study in the hippocampus. Brain Res. 265, 21–thirty. doi: 10.1016/0006-8993(83)91329-x
CrossRef Full Text | Google Scholar
Dilly, P. Northward., Grey, Due east. G., and Young, J. Z. (1963). Electron microscopy of optic nerves and optic lobes of Octopus and Eledone. Proc. R. Soc. Ser. B-Bio. 158, 446–456. doi: ten.1098/rspb.1963.0057
PubMed Abstract | CrossRef Total Text | Google Scholar
Frotscher, M., and Leranth, C. (1986). The cholinergic innervation of the rat fascia-dentata - identification of target structures on granule cells by combining choline-acetyltransferase immunocytochemistry and Golgi impregnation. J. Comp. Neurol. 243, 58–70. doi: 10.1002/cne.902430106
PubMed Abstract | CrossRef Full Text | Google Scholar
Ganeshina, O., Drupe, R. Due west., Petralia, R. S., Nicholson, D. A., and Geinisman, Y. (2004a). Synapses with a segmented, completely partitioned postsynaptic density express more than AMPA receptors than other axospinous synaptic junctions. Neuroscience 125, 615–623. doi: 10.1016/j.neuroscience.2004.02.025
PubMed Abstract | CrossRef Full Text | Google Scholar
Ganeshina, O., Berry, R. W., Petralia, R. S., Nicholson, D. A., and Geinisman, Y. (2004b). Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J. Comp. Neurol. 468, 86–95. doi: x.1002/cne.10950
PubMed Abstract | CrossRef Full Text | Google Scholar
Gardner, C. 50., Jones, J. R., Baer, S. Grand., and Cheat, South. M. (2015). Drift-diffusion simulation of the ephaptic effect in the triad synapse of the retina. J. Comput. Neurosci. 38, 129–142. doi: x.1007/s10827-014-0531-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Gruber, Fifty., Rybak, J., Hansson, B. S., and Cantera, R. (2018). Synaptic spinules in the olfactory excursion of Drosophila melanogaster. Front. Cell Neurosci. 12:86. doi: x.3389/fncel.2018.00086
PubMed Abstruse | CrossRef Total Text | Google Scholar
Guangming, G., Junhua, G., Chenchen, Z., Yang, M., and Wei, 10. (2020). Neurexin and neuroligins maintain the balance of ghost and satellite boutons at the Drosophila neuromuscular junction. Front. Neuroanat. xiv:00019. doi: x.3389/fnana.2020.00019
PubMed Abstract | CrossRef Full Text | Google Scholar
Haghighat, Northward., Cohen, R. S., and Pappas, G. D. (1984). Fine-structure of squid (Loligo-pealei) optic lobe synapses. Neuroscience 13, 527–546. doi: x.1016/0306-4522(84)90246-x
CrossRef Full Text | Google Scholar
Hall, D. H., and Russell, R. L. (1991). The posterior nervous-system of the nematode Caenorhabditis elegans - serial reconstruction of identified neurons and consummate pattern of synaptic-interactions. J. Neurosci. xi, 1–22. doi: 10.1523/jneurosci.11-01-00001.1991
PubMed Abstract | CrossRef Full Text | Google Scholar
Henze, D. A., Urban, N. N., and Barrionuevo, Thou. (2000). The multifarious hippocampal mossy fiber pathway: a review. Neuroscience 98, 407–427. doi: 10.1016/s0306-4522(00)00146-nine
CrossRef Full Text | Google Scholar
Hirano, A. A., Vuong, H. E., Kornmann, H. L., Schietroma, C., Stella, Southward. L., Barnes, S., et al. (2020). Vesicular release of GABA by mammalian horizontal cells mediates inhibitory output to photoreceptors. Front. Prison cell Neurosci. 14:600777. doi: x.3389/fncel.2020.600777
PubMed Abstract | CrossRef Total Text | Google Scholar
Kramer, R. H., and Davenport, C. M. (2015). Lateral inhibition in the vertebrate retina: The case of the missing neurotransmitter. PLoDue south Biol. 13:1002322. doi: ten.1371/journal.pbio.1002322
PubMed Abstract | CrossRef Full Text | Google Scholar
Linberg, Grand. A., and Fisher, S. M. (1988). Ultrastructural Evidence That horizontal cell axon terminals are presynaptic in the human retina. J. Comp. Neurol. 268, 281–297. doi: 10.1002/cne.902680211
PubMed Abstract | CrossRef Full Text | Google Scholar
Liu, X., Hirano, A. A., Sunday, X. P., Brecha, N. C., and Barnes, Southward. (2013). Calcium channels in rat horizontal cells regulate feedback inhibition of photoreceptors through an unconventional GABA- and pH-sensitive mechanism. J. Physiol-Lon. 591, 3309–3324. doi: 10.1113/jphysiol.2012.248179
PubMed Abstract | CrossRef Full Text | Google Scholar
Maddox, J. Westward., Randall, Grand. I., Yadav, R. P., Williams, B., Hagen, J., and Derr, P. J. (2020). A dual role for Ca v 1.iv Ca2+ channels in the molecular and structural arrangement of the rod photoreceptor synapse. eLife 9:62184. doi: x.7554/eLife.62184
PubMed Abstract | CrossRef Full Text | Google Scholar
Mitchell, N., Petralia, R. S., Currier, D. Thousand., Wang, Y. Ten., Kim, A., Mattson, One thousand. P., et al. (2012). Sonic hedgehog regulates presynaptic concluding size, ultrastructure and role in hippocampal neurons. J. Cell Sci. 125, 4207–4213. doi: ten.1242/jcs.105080
PubMed Abstract | CrossRef Total Text | Google Scholar
Nagerl, U. Five., Willig, K. I., Hein, B., Hell, S. Due west., and Bonhoeffer, T. (2008). Live-cell imaging of dendritic spines by STED microscopy. P. Natl. Acad. Sci. USA 105, 18982–18987. doi: x.1073/pnas.0810028105
PubMed Abstract | CrossRef Total Text | Google Scholar
Nilsson, D. E., Gislen, L., Coates, Grand. M., Skogh, C., and Garm, A. (2005). Advanced optics in a jellyfish eye. Nature 435, 201–205. doi: 10.1038/nature03484
PubMed Abstract | CrossRef Full Text | Google Scholar
Omiya, Y., Uchigashima, M., Konno, M., Yamasaki, Yard., Miyazaki, T., Yoshida, T., et al. (2015). VGluT3-expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in detail cortical and cortex-like amygdaloid regions of mouse brains. J. Neurosci. 35, 4215–4228. doi: 10.1523/jneurosci.4681-14.2015
PubMed Abstruse | CrossRef Full Text | Google Scholar
Pelzer, P., Horstmann, H., and Kuner, T. (2017). Ultrastructural and functional properties of a giant synapse driving the piriform cortex to mediodorsal thalamus project. Front end. Synaptic. Neurosci. 9:three. doi: x.3389/fnsyn.2017.00003
PubMed Abstract | CrossRef Full Text | Google Scholar
Petralia, R. S., Mattson, Yard. P., and Yao, P. J. (2014). Communication breakdown: The bear on of ageing on synapse structure. Ageing Res. Rev. 14, 31–42. doi: 10.1016/j.arr.2014.01.003
PubMed Abstruse | CrossRef Full Text | Google Scholar
Petralia, R. S., Wang, Y. X., Mattson, M. P., and Yao, P. J. (2015). Structure, distribution, and part of neuronal/synaptic spinules and related invaginating projections. Neuromol. Med. 17, 211–240. doi: 10.1007/s12017-015-8358-vi
PubMed Abstract | CrossRef Full Text | Google Scholar
Petralia, R. Southward., Wang, Y. Ten., Mattson, M. P., and Yao, P. J. (2017). Invaginating presynaptic terminals in neuromuscular junctions, photoreceptor terminals, and other synapses of animals. Neuromol. Med. nineteen, 193–240. doi: 10.1007/s12017-017-8445-y
PubMed Abstract | CrossRef Full Text | Google Scholar
Rigon, F., Gasparini, F., Shimeld, South. M., Candiani, Southward., and Manni, L. (2018). Developmental signature, synaptic connectivity and neurotransmission are conserved betwixt vertebrate hair cells and tunicate coronal cells. J. Comp. Neurol. 526, 957–971. doi: 10.1002/cne.24382
PubMed Abstract | CrossRef Full Text | Google Scholar
Rodriguez-Moreno, J., Rollenhagen, A., Arlandis, J., Santuy, A., Merchan-Pérez, A., DeFelipe, J., et al. (2018). Quantitative 3D ultrastructure of thalamocortical synapses from the "lemniscal" ventral posteromedial nucleus in mouse barrel Cortex. Cereb. Cortex 28, 3159–3175. doi: x.1093/cercor/bhx187
PubMed Abstract | CrossRef Full Text | Google Scholar
Rodriguez-Moreno, J., Porrero, C., Rollenhagen, A., Rubio-Teves, M., Casas-Torremocha, D., and Alonso-Nanclares, L. (2020). Surface area-specific synapse construction in branched posterior nucleus axons reveals a new level of complexity in thalamocortical networks. J. Neurosci. 40, 2663–2679. doi: 10.1523/jneurosci.2886-19.2020
PubMed Abstract | CrossRef Full Text | Google Scholar
Roelandse, Chiliad., Welman, A., Wagner, U., Hagmann, J., and Matus, A. (2003). Focal motility determines the geometry of dendritic spines. Neuroscience 121, 39–49. doi: 10.1016/s0306-4522(03)00405-6
CrossRef Full Text | Google Scholar
Rollenhagen, A., Walkenfort, B., Yakoubi, R., Klauke, S. A., Schmuhl-Giesen, S. F., and Heinen-Weiler, J. (2020). Synaptic organization of the homo temporal lobe neocortex as revealed by high-resolution transmission, focused ion axle scanning, and electron microscopic tomography. Int. J. Mol. Sci. 21:5558. doi: 10.3390/ijms21155558
PubMed Abstruse | CrossRef Full Text | Google Scholar
Stark, West. Southward., Sapp, R., and Carlson, S. D. (1989). Ultrastructure of the ocellar visual-arrangement in normal and mutant Drosophila melanogaster. J. Neurogenet. 5, 127–153. doi: x.3109/01677068909066203
PubMed Abstract | CrossRef Total Text | Google Scholar
Tao-Cheng, J. H., Dosemeci, A., Gallant, P. E., Miller, S., Galbraith, J. A., Winters, C. A., et al. (2009). Rapid turnover of spinules at synaptic terminals. Neuroscience 160, 42–50. doi: x.1016/j.neuroscience.2009.02.031
PubMed Abstract | CrossRef Total Text | Google Scholar
Tsukamoto, Y., Iseki, Thousand., and Omi, Northward. (2021). Helical fasciculation of bipolar and horizontal cell neurites for wiring with photoreceptors in macaque and mouse retinas. Invest. Ophthalmol. Vis. Sci. 62:31. doi: ten.1167/iovs.62.one.31
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ueda, Y., and Hayashi, Y. (2013). PIP3 regulates spinule formationin dendritic spines during structured long-term potentiation. J. Neurosci. 33, 11040–11047. doi: 10.1523/jneurosci.3122-12.2013
PubMed Abstract | CrossRef Full Text | Google Scholar
Van Vactor, D., and Sigrist, S. J. (2017). Presynaptic morphogenesis, active zone arrangement and structural plasticity in Drosophila. Curr. Opin. Neurobiol. 43, 119–129. doi: 10.1016/j.conb.2017.03.003
PubMed Abstruse | CrossRef Total Text | Google Scholar
Wang, Y. C., Fehlhaber, Thousand. E., Sarria, I., Cao, Y., Ingram, N. T., and Guerrero-Given, D. (2017). The auxiliary calcium aqueduct subunit alpha ii delta 4 is required for axonal elaboration, synaptic manual, and wiring of rod photoreceptors. Neuron 93, 1359–1374. doi: 10.1016/j.neuron.2017.02.021
PubMed Abstruse | CrossRef Full Text | Google Scholar
Ware, R. W., Clark, D., Crossland, K., and Russell, R. L. (1975). Nerve ring of nematode Caenorhabditis elegans - Sensory input and motor output. J. Comp. Neurol. 162, 71–110. doi: ten.1002/cne.901620106
CrossRef Full Text | Google Scholar
Weiler, R., and Schultz, Thousand. (1993). Ionotropic Non-N-methyl-D-aspartate agonists induce retraction of dendritic spinules from retinal horizontal cells. P. Natl. Acad. Sci. The states xc, 6533–6537. doi: x.1073/pnas.90.14.6533
PubMed Abstract | CrossRef Total Text | Google Scholar
Westrum, L. E., and Blackstad, T. W. (1962). Electron microscopic report of stratum radiatum of rat hippocampus (regio superior, Ca ane) with detail accent on synaptology. J. Comp. Neurol. 119, 281–309. doi: x.1002/cne.901190303
PubMed Abstract | CrossRef Total Text | Google Scholar
White, J. G., Southgate, E., Thomson, J. N., and Brenner, S. (1986). The structure of the nervous-organisation of the nematode Caenorhabditis elegans. Philos. T. Roy. Soc. B 314, ane–340. doi: ten.1098/rstb.1986.0056
PubMed Abstract | CrossRef Full Text | Google Scholar
Wood, B. M., Baena, V., Huang, H., Jorgens, D. M., Terasaki, Thou., and Kornberg, T. B. (2021). Cytonemes with circuitous geometries and limerick extend into invaginations of target cells. J. Cell Biol. 220:202101116. doi: x.1083/jcb.202101116
PubMed Abstract | CrossRef Full Text | Google Scholar
Yao, P. J., Eren, E., Petralia, R. S., Gu, J. W., Wang, Y. X., and Kapogiannis, D. (2020). Mitochondrial protrusions in neuronal cells. Iscience 23:101514. doi: x.1016/j.isci.2020.101514
PubMed Abstruse | CrossRef Total Text | Google Scholar
Yao, P. J., Petralia, R. S., Bushlin, I., Wang, Y., and Furukawa, Grand. (2005). Synaptic distribution of the endocytic accessory proteins AP180 and CALM. J. Comp. Neurol. 481, 58–69. doi: 10.1002/cne.20362
PubMed Abstruse | CrossRef Full Text | Google Scholar
Yoshida, T., Uchigashima, M., Yamasaki, M., Katona, I., Yamazaki, Thousand., Sakimura, K., et al. (2011). Unique inhibitory synapse with specially rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. P. Natl. Acad. Sci. USA 108, 3059–3064. doi: x.1073/pnas.1012875108
PubMed Abstract | CrossRef Total Text | Google Scholar
Zaccard, C. R., Shapiro, Fifty., Martin-de-Saavedra, Grand. D., Pratt, C., Myczek, K., Song, A., et al. (2020). Rapid 3D enhanced resolution microscopy reveals variety in dendritic spinule dynamics, regulation, and function. Neuron 107, 522–537. doi: ten.1016/j.neuron.2020.04.025
PubMed Abstract | CrossRef Total Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fnsyn.2021.685052/full
0 Response to "Structure and Location for Synapse - Lab Review"
Post a Comment